Battery Components: Detailed Structure and Operation
Inside every battery that powers our vehicles and devices lies a complex electrochemical ecosystem that allows for the efficient storage and delivery of electrical energy. From the first start of the engine to the operation of the air conditioning, these accumulators depend on the precise interaction between multiple internal components working in perfect synchronization. A battery is, in technical terms, a rechargeable electrochemical device, commonly known as a battery, and is essential in applications such as automobiles, trucks, and other industrial uses. Furthermore, the battery manufacturing process involves the careful selection of materials and components, which determines their performance and durability. Each of these elements performs a specific function within the electrochemical system, ensuring the proper functioning of the entire system. Cars and other vehicles rely on batteries for multiple electrical functions, from starting to powering auxiliary systems.

Historically, the term "battery" was used to designate the earliest electrical energy storage devices, differentiating between primary (non-rechargeable) and secondary (rechargeable) cells. It is important to distinguish between batteries, accumulators, and cells: a battery typically refers to a set of connected cells, an accumulator to rechargeable devices, and a cell to non-rechargeable devices; each is used in specific contexts, such as automobiles, cars, or portable devices. Through this detailed analysis, we will explore each element that makes the conversion of chemical energy into electrical energy possible, from the plates to the most advanced safety systems. It is worth noting that batteries come in a variety of shapes and sizes, adapting to different applications and technologies.
Finally, it's crucial to choose quality batteries to ensure the proper functioning of electrical systems and extend the lifespan of devices. It's essential to understand the battery's components, the energy sources involved in its operation, and have accurate technical information to make informed decisions and ensure safe and efficient use.
Comcast Group: Presence and Product Line in Mexico and Central America
Comcast Group has established itself as a leader in Mexico and Central America in the distribution of reliable and efficient energy solutions. With a focus on innovation and quality, it offers a wide range of batteries and inverters designed to meet a variety of residential, commercial, and industrial needs.
Battery Line
Comcast Group's battery offering ranges from 50 Ah to 200 Ah, ideal for applications requiring long-lasting and stable energy storage. These batteries are designed to provide continuous and reliable power, withstanding extended charge and discharge cycles, with excellent service life and resistance to adverse conditions.
To learn more about available batteries, visit the official website:Comcast Group Batteries .
Investor Line
Comcast Group also offers inverters with power ratings ranging from 3 kVA to 10 kVA, capable of converting stored energy into alternating current to power everything from small appliances to complete electrical systems. These inverters stand out for their energy efficiency, low noise levels, and ability to operate in a variety of environments.
See the full line of inverters at: Comcast Group Investors .
Technical Specifications
- Power: 50 to 200 Ah batteries; 3 to 10 kVA inverters, covering a wide range of energy needs.
- Operating time: Depends on battery capacity and connected load; designed to provide extended runtime in residential and commercial applications. Service batteries are specifically designed for applications requiring deep, continuous charge and discharge cycles, which are different from conventional automotive batteries.
- Noise Levels: The inverters feature silent technology that minimizes operating noise, ensuring a comfortable environment.
Real-Life Use Cases
Comcast Group solutions are ideal for:
- Electrical backup systems in homes and offices.
- Photovoltaic solar installations for the storage and supply of clean energy.
- Medical equipment and critical systems that require uninterrupted power.
- Industrial and commercial projects that demand high capacity and reliability.

Shipping Options and Payment Plans
Comcast Group offers secure and fast shipping throughout Mexico and Central America, with online tracking options for customer peace of mind.
To facilitate purchasing, various payment methods are accepted, including PayPal, Mercado Pago, and Oxxo store payments, adapting to local preferences and ensuring convenient and secure transactions.
Warranty and Support
All products come with a one-year warranty that supports their quality and performance. Additionally, Comcast Group offers personalized support via WhatsApp, allowing direct and efficient communication to resolve questions, provide technical advice, and address any issues.
Customer Testimonials
Satisfied customers praise Comcast Group's durability, efficiency, and customer service:
"Comcast Group batteries have been instrumental in keeping our operations running smoothly, and WhatsApp support is fast and effective." – Juan P., Mexico City.
"I purchased a 10 kVA inverter and the installation was simple. The unit operates quietly and reliably." – María L., Guadalajara.
Call to Action
If you're looking for reliable energy solutions tailored to your needs in Mexico and Central America, don't hesitate to visit our online store and discover the wide range of batteries and inverters Comcast Group has for you.
Explore our entire offering : Energy and Batteries Comcast Group .
Secure your electricity supply today with the quality and support that only Comcast Group can offer!
What is a battery and what are its basic elements?
A battery is an electrochemical device that transforms chemical energy into electrical energy through oxidation and reduction reactions between two electrodes immersed in an electrolyte. This process occurs when electrons flow from the anode (negative electrode) to the cathode (positive electrode) through an external circuit, while ions migrate within the battery to maintain the balance of electrical charges.
The components of a battery are mainly composed of three fundamental elements:
- Anode (negative electrode): This is the part where oxidation occurs, releasing electrons that flow to the external circuit.
- Cathode (positive electrode): Reduction occurs here, receiving electrons from the circuit.
- Electrolyte: A conductive medium that allows the movement of ions between electrodes, facilitating internal chemical reactions.
Electrodes are typically made of a mixture of different materials, such as lead oxides and sulfates, which influence the battery's function and performance. Additionally, some internal components, such as separators and containers, are made of plastic due to its strength and durability under harsh conditions.

These elements work together during the charging and discharging processes to store and release electrical energy. There are different products on the market, such as batteries, which vary in size, composition, and applications, adapting to diverse energy needs. Secondary cells, unlike primary cells, can be recharged and discharged multiple times, which increases their durability and reduces their environmental impact.
Difference between primary and secondary cells
Batteries are classified according to their recharge capacity into:
- Primary cells (non-rechargeable): Like alkaline batteries, where chemical reactions are irreversible. These can only be used once and must be discarded after use, as their chemical structure changes permanently.
- Secondary cells (rechargeable): Such as lead-acid or lithium batteries, which allow the flow of electrons to be reversed when connected to an external electrical source, restoring their energy capacity through reversible chemical reactions. The number of charge and discharge cycles directly affects the lifespan of these batteries.
Positive Electrode (Cathode)
The cathode is the positive electrode where electrons are received during discharge, undergoing a chemical reduction process. It is made of specific compounds selected for their ability to withstand electrochemical reactions and provide good electrical conductivity. The composition of the cathode varies depending on the technology used, determining characteristics such as voltage, capacity, and battery life.
Materials according to battery type
In a lead-acid battery, the cathode is composed of lead dioxide (PbO₂) on a metal grid, offering excellent conductivity and stability in the acidic medium of the electrolyte.
Lithium-ion batteries use different metal oxides depending on the application:
- Lithium cobalt oxide (LiCoO₂): High energy density for electronic devices.
- Lithium iron phosphate (LiFePO₄): Greater thermal safety for electric vehicles.
- Lithium manganese oxide (LiMn₂O₄): Balancing cost and performance.
In nickel-cadmium (Ni-Cd) batteries, the cathode is composed of nickel(III) hydroxide (NiOOH), while NiMH batteries use similar compositions optimized for higher capacity.

Reduction process at the cathode
During discharge, the cathode receives electrons from the external circuit, while positive ions from the electrolyte migrate toward it. This reduction reaction releases the chemically stored energy, allowing direct current to flow to the connected device.
The choice of cathode material directly influences the cell's nominal voltage, energy density, and safety characteristics. For example, cobalt-based cathodes provide high energy density but lower thermal stability compared to iron phosphate variants.
Negative Electrode (Anode)
The anode is the negative electrode that acts as a source of electrons during discharge, undergoing oxidation and releasing electrons into the external circuit. Its design and composition determine the storage capacity and efficiency of charge and discharge cycles, influencing the battery's lifespan and performance.
Anode composition by technology
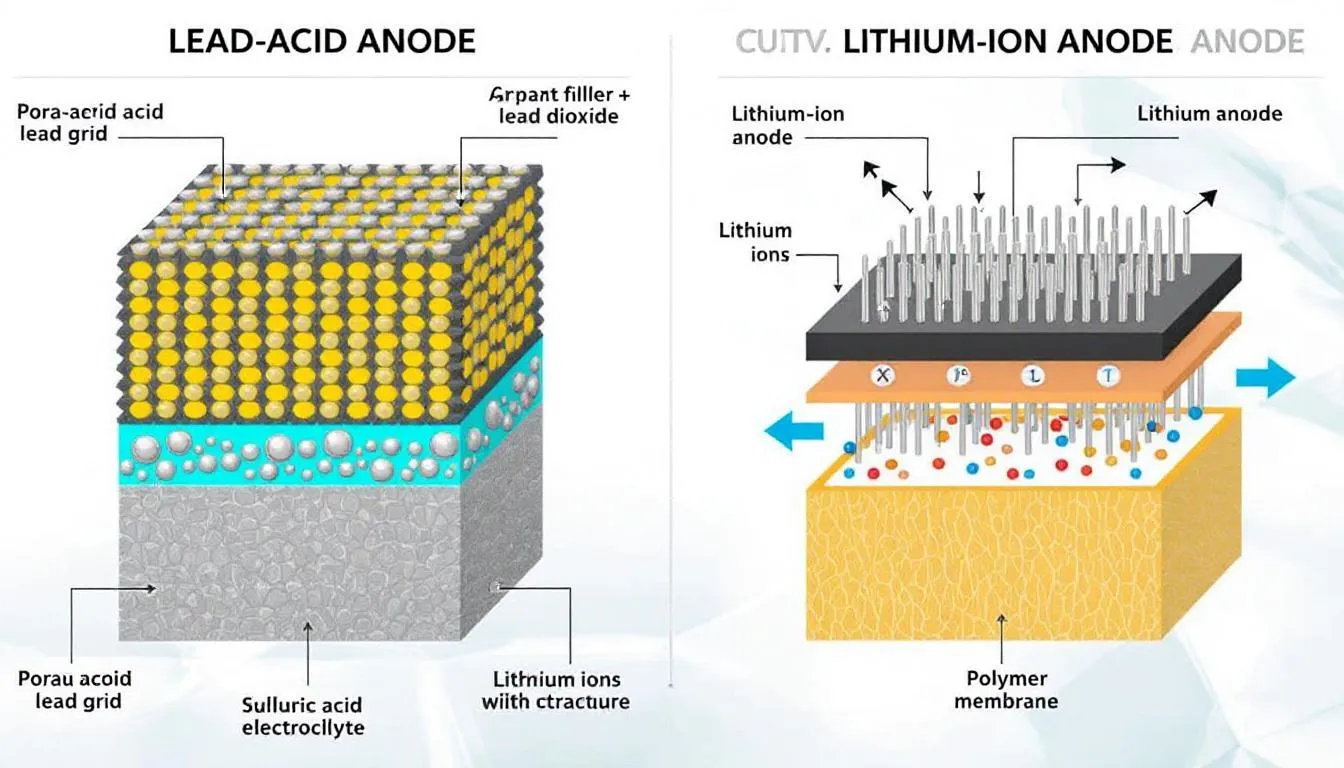
In lead-acid batteries, the anode is made of spongy or porous lead mounted on support grids, maximizing the contact surface with the electrolyte to optimize chemical reactions.
In lithium batteries, graphite is used as an anode material due to its ability to reversibly intercalate lithium ions during charging and discharging, without degrading its crystalline structure.
Function as an energy store
During charging, the anode stores ions, while during discharging it releases them along with electrons. This process must be highly reversible to ensure a long battery life.
In lead-acid batteries, special additives are added to prevent sulfation, the buildup of lead sulfate crystals that reduces capacity. These additives improve electrical conductivity and crystal structure, especially at low temperatures where performance is often reduced.
Electrolyte
The electrolyte is the ion-conducting medium that electrically connects the electrodes, facilitating the redox reactions that generate electricity. Its chemical composition varies depending on the technology, affecting the battery's performance and lifespan.
Composition according to technology
- Lead-acid batteries: These use a solution of sulfuric acid diluted in distilled water, with a concentration that varies depending on the state of charge. A higher percentage of sulfuric acid indicates a higher stored charge.
- Lithium-ion batteries: These use solutions of lithium salts dissolved in organic carbonate mixtures, preventing water electrolysis and allowing higher voltages.
- Alkaline cells (Ni-Cd, NiMH): They use potassium hydroxide (KOH) in aqueous solution for high ionic conductivity in a basic medium.
Role in electrochemical reactions
The electrolyte allows the free movement of ions between the anode and cathode, closing the internal circuit and facilitating the electrochemical reaction. During discharge, ions migrate from the anode to the cathode through the electrolyte, and during charging, the process is reversed.
The concentration and density of the electrolyte affect ionic conductivity, reaction rate, total capacity, and battery life. In lead-acid batteries, electrolyte density can be measured with hydrometers to assess the state of charge.
Innovations in electrolytes
Gel electrolytes, used in sealed AGM batteries, immobilize sulfuric acid in a gel matrix. This minimizes the risk of spillage, reduces evaporation, and improves vibration resistance, making them ideal for modern automotive applications.
Support Grids
The support grids form the structure that physically and electrically supports the active material of the plates, acting as a conductive skeleton that distributes the electric current evenly across the entire electrode surface. The cross-section of the conductors in these grids determines the amount of current that can flow without overheating, directly influencing the battery's charging capacity.
Materials and alloys
Traditionally, grids are made of lead with the addition of other metals to optimize their properties:
- Antimony alloys: They provide mechanical strength, but increase self-discharge and require frequent maintenance.
- Calcium alloys: Reduce self-discharge and gas generation, resulting in low-maintenance batteries.
- Advanced alloys: Incorporate tin, silver or other elements to improve conductivity, corrosion resistance and durability.
Structural and electrical function
The grid serves critical functions such as mechanical support, current conduction to external terminals, uniform distribution of electrical flow, and resistance to thermal and chemical stresses. Its design influences internal resistance and ability to deliver starting current, especially in vehicles.
Separators
The separator physically isolates the positive and negative plates to prevent short circuits, while allowing ions from the electrolyte to pass through. It is made of tough, flexible, and durable materials capable of withstanding extreme conditions inside the battery.
Materials and characteristics
- Microporous polyethylene: Standard material in lead-acid batteries, resistant to sulfuric acid and stable at high temperatures.
- Fiberglass (AGM): Completely absorbs the electrolyte, eliminating free liquid and improving efficiency in charge and discharge cycles.
- Ceramic materials: Used in high temperature applications to maintain structural integrity.
Critical properties
Separators must have mechanical strength to withstand internal pressures, chemical stability against the electrolyte, low ionic resistance to minimize impedance, and thermal stability to operate over wide temperature ranges. The development of advanced materials seeks to maximize lifespan and withstand conditions such as overloads, vibrations, and high temperatures.
Container and Lid
The container and lid form the protective packaging that contains all the internal components, isolating them from the environment and protecting them from impacts, chemicals, and adverse conditions. In addition to polypropylene, other plastics are used in the manufacture of containers and lids due to their strength, lightness, and durability in cold temperatures and exposure to chemical liquids.
Construction material
Polypropylene is the predominant material due to its resistance to sulfuric acid, immunity to automotive fuels, low weight, and ease of molding to create complex internal compartments.
Structural design
The vessel is divided into internally connected compartments or cells, each with a set of positive and negative plates. Settling chambers at the bottom collect sediment to prevent short circuits.
The cover may include connection terminals, vent valves for pressure control, visual charge status indicators, and filling systems for maintainable batteries.
Recycling Considerations
The use of polypropylene allows for efficient separation during recycling, contributing to the circular economy and reducing the environmental impact at the end of the battery's useful life.
Safety and Ventilation Elements
Safety systems are essential for safe battery operation, considering the generation of gases during charging and possible overload or internal failure. Some batteries are designed to withstand repeated discharge and recharge cycles, ideal for applications requiring greater durability and performance.
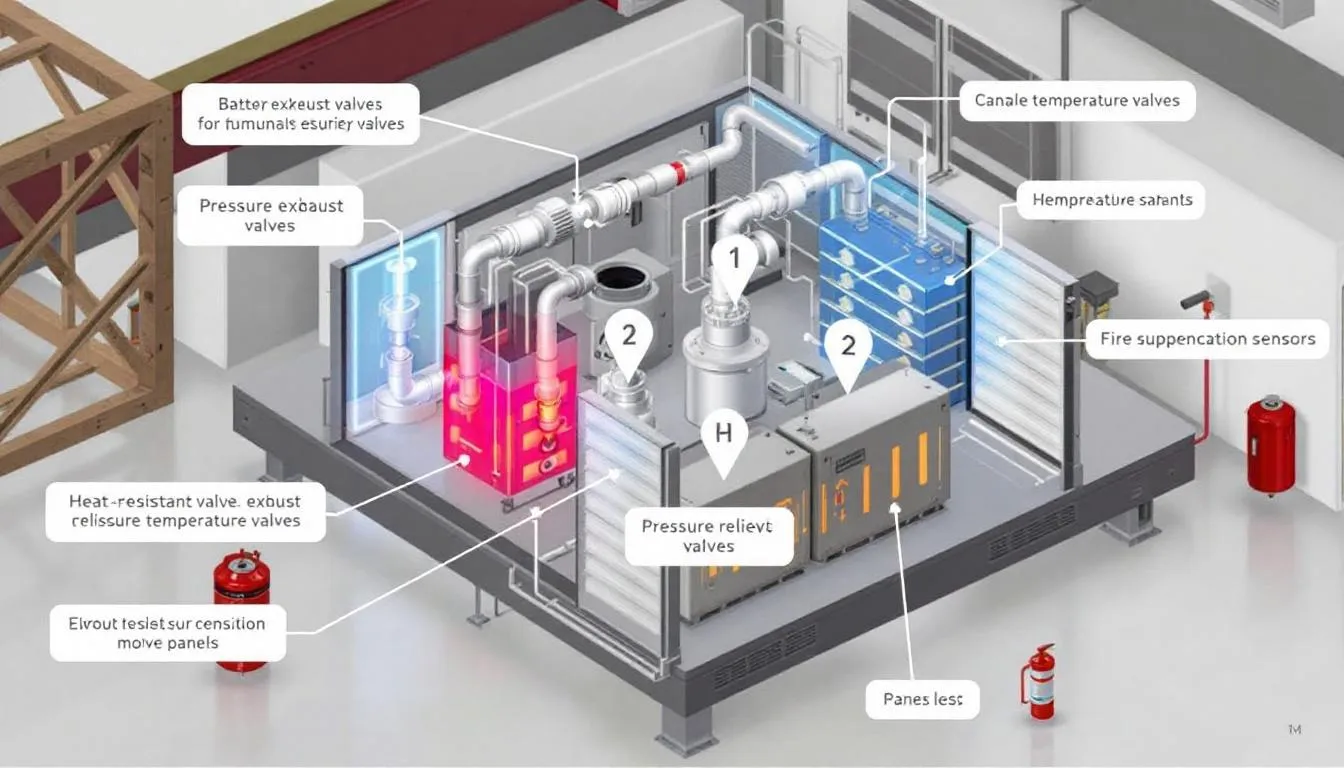
Vent valves
During charging, especially in the final phase, water electrolysis occurs, generating hydrogen and oxygen. Valves release these gases in a controlled manner to prevent dangerous pressure buildup, the risk of explosion, and structural damage.
In conventional batteries, removable caps allow for checking electrolyte levels and maintenance. Sealed batteries incorporate one-way valves that only open when pressure exceeds safe limits.
Status indicators
Visual indicators, such as built-in hydrometers ("magic eyes"), show the charging status through color changes on a floating dial. Green indicates a full charge, black indicates a discharge, and white indicates a low electrolyte level.
Advanced electronic systems monitor voltage, temperature and current, connected to battery management systems (BMS) that prevent dangerous conditions.
Overload protection
Modern systems include protections against overcharging, deep discharge, short circuits, and excessive temperature, which are essential in lithium batteries to avoid risks such as thermal runaway.
Types of Components according to Battery Technology
Components vary by technology, each optimized for specific applications with particular performance, safety, and cost requirements. In automobiles, the battery is key to the start-up and operation of electrical systems. Furthermore, batteries supply power to the electric and combustion engines, which are critical for vehicle propulsion and determine its efficiency and sustainability.
Lead-Acid Batteries
- Conventional (flooded): Free liquid electrolyte, lead plates, and polyethylene separators. They require maintenance but have a low initial cost.
- AGM (Absorbed Glass Mat): Electrolyte absorbed in fiberglass, greater resistance to vibrations and deep cycle.
- Gel: Gelled electrolyte, ideal for deep cycling and extreme temperatures.
- EFB (Enhanced Flooded Battery): Reinforced plates and additives for greater charge acceptance, used in start-stop vehicles.
Lithium-ion batteries
They replace traditional materials with graphite anodes, metal oxide cathodes, and non-aqueous organic electrolytes, with mandatory safety management systems.
Performance comparison
|
Technology |
Energy Density |
Life Cycles |
Maintenance |
Initial Cost |
|---|---|---|---|---|
|
Lead-Acid |
Low |
300-500 |
High |
Low |
|
AGM |
Medium-Low |
400-600 |
Minimum |
Half |
|
Lithium |
High |
1000-3000+ |
None |
High |
|
NiMH |
Average |
500-1000 |
Low |
Medium-High |
Lithium batteries offer greater energy density and lifespan, but require sophisticated systems. Lead-acid batteries maintain advantages in cost, recyclability, and simplicity.
Maintenance and Care of Components
The deterioration of internal components is the main cause of failure, so proper preventive maintenance maximizes useful life and performance.

Inspection of terminals and connections
Terminal corrosion is common and affects performance. Cleaning with baking soda, a wire brush, and dielectric grease is recommended. Inspection should be performed monthly in humid environments.
Electrolyte monitoring
In batteries requiring maintenance, verify that the electrolyte level covers the plates and measure the density with a hydrometer to assess the charge. Use only distilled water for refilling.
External cleaning
Remove dirt and acid residue to prevent stray currents that drain the battery and improve heat dissipation.
Identification of deterioration
Detect swelling, leaks, loose terminals, or poor performance for timely replacement. Perform idle and load voltage tests.
Correct loading procedures
Use slow charging at 10% capacity for a full charge, controlled rapid charging, and maintenance charging to compensate for self-discharge. Avoid overcharging to prevent damage.
Current and Future Perspective
The battery industry is advancing rapidly, driven by electromobility, renewable energy, and the demand for efficient and sustainable devices.
Emerging technological trends
- Solid-state batteries: They replace liquid electrolyte with solid materials, increasing energy density and safety.
- Cobalt-free chemistry: To reduce costs and ethical issues, alternatives such as LiFePO₄ are being investigated.
- Advanced recycling: Processes that recover up to 95% of valuable materials, promoting a circular economy.
Digitalization and intelligence
Management systems are evolving toward intelligent platforms with predictive algorithms, IoT connectivity, and digital twins to optimize maintenance and performance.
Emerging applications
- Stationary storage: To stabilize electrical grids with renewable energy.
- Air Mobility: Ultralight Batteries for Drones and Electric Aviation.
- Portable Devices: Miniaturization for Wearables and Medical Implants.
The future of battery components is moving toward more sustainable materials, intelligent systems, and diverse applications, while maintaining a balance between performance, safety, cost, and environmental impact.


